by Shane L. Larson
Friends and family who travel around with me know I have a fatal weakness for one of the most ephemeral manifestations of the human brain: museums. Museums ostensibly exist for the singular purpose of capturing and showing what we as a species have learned, what we have discovered, and what still gives us wonder about the vast and mysterious world around us.

A “Little Free Library,” one of the modern forms of libraries, found in neighborhoods around the world.
In many ways, they are like their sister institutions, an equally ephemeral result of our unique brains: libraries. Libraries ostensibly exist for the singular purpose of storing the knowledge our species has accumulated, dispersed freely and at will to anyone who walks through the doors. I have a fatal weakness for libraries as well.
What do I mean by fatal weakness? I mean if I walk into one of these spaces, I’m consumed by it. In a museum, I linger and dwell at every exhibit, I read the detailed descriptions, I go back to previous exhibits to see how it is all connected. In a library, I walk down the aisles brushing my fingers lightly over the spines of books, drinking in the titles, sometimes pulling one off the shelf to thumb through the pages.
Every now and then, I ponder why we first decided to create these museums and libraries. Often when people think about museums and attempt to describe them, they describe the things there: artifacts, rocks, shards of lost civilizations, exquisite pieces of art, stuffed creatures that once roamed the wilds.
Consider what you might see if you visit the Adler Planetarium in Chicago. There you can see a star show on the planetarium dome, touch a fragment of the dwarf planet Ceres with your own bare hand, see a life-size model of the Opportunity rover on Mars, and stand next to the Gemini 12 capsule that carried Jim Lovell and Buzz Aldrin around the Earth for almost four days in 1966.
But these are all things, and while the things are a focal point that draws you into the museum, they are not why you are there. You stare, and linger, and imagine something quite different and ephemeral. What is that?
Dr. Michelle Larson, the President of the Adler, describes this dichotomy as “nouns” and “verbs.” The nouns are what attract our attention, but what we are looking for and hoping to find are verbs.
Standing in front of Gemini 12, a thin pane of plexiglass keeping you barely a half meter away, you reach out your hand. What are you doing? Hoping to verify the construction of aluminum? Check that the paint is peeling? No, much more.

Closeups of the Gemini 12 capsule, showing the cramped space of the crew cabin (left), and the scorched heatshield (right).
The almost involuntary movement of your hand is because your brain is imagining what it was like to hurtle through the vacuum of space at nearly 28,000 kilometers per hour (more than 17,000 miles per hour), protected by the thinnest veneer of metal and insulation — was it terrifying or exhilarating or both? You look at the tiny window, and try to imagine seeing the color and light that was seen as the capsule plummeted down to Earth on the way home. The blast pattern of char on the back of Gemini 12, where the heat shield protected Lovell and Aldrin from the 2700 degree Celsius inferno, makes you wonder: if someone touched the window would it feel warm? Staring at the tiny confined space where the astronauts lived for almost four days you wonder: did it smell in there?
The Gemini 12 capsule, the noun, is just a vehicle to stimulate your thinking about the experiences, the verbs.
This spills over into hobbies. Consider birdwatching. For some of us, birds are sortable at best into “robins,” “ducks,” and “little brown birds.” But for people who identify as birders, there is a certain unconstrained joy that people find in seeing the widest possible variety of our feathered friends as they can. They meticulously stare at birds through binoculars in the backyard, slog down park trails to remote copses of trees, and diligently put food and water in the backyard, all to be afforded a chance to see a blue-winged teal, or an eastern meadowlark, or a tufted tit-mouse.

A collection of birds from the winter in Illinois. A bluejay (top left), a tufted titmouse (lower left), and a cardinal (right). [Photos: M. Larson]
The birds are nouns, but birders aren’t collecting birds. They are collecting the experience of seeing birds, the verbs of birding. Birding is a verb! The joy is seeing the delicate splash of color of feathers iridescent in the sun, of projecting your own joy on a bluejay who looks thrilled to have a peanut in its mouth, or hearing a mother cardinal squawking at her fledglings encouraging them to take wing for the first time.

Looking at the Moon through a telescope or binoculars always fires the imagination. [Photo: S. Larson]
Amateur astronomers are the same way. We stand out in the backyard, hunkered down in our winter jackets against the cold, peering intently into the eyepiece of a telescope, straining to see photons that have spent two million years sailing the void from the Andromeda galaxy to Earth. The telescopes, the photons themselves, are just nouns. They are cool things unto themselves. But the astronomer is experiencing the light, drowning themselves in the existential awe of imagining the enormous gulf that photon has crossed to ultimately fall into their eye. Perhaps the light originated right next door, on the Moon, or perhaps it started its journey long, long ago far across the Cosmos in a star in a galaxy so far away humans haven’t named it. That light journeyed for longer than humans have been on Earth, and ended its voyage rattling through a few telescope mirrors and terminating on the retina of an eyeball. Imagine the journey that light took.
All of the practice of science can be thought of in this way: it’s nouns and verbs. The nouns are the things you get taught, that you can look up on Wikipedia, that you hear about on the news. Science is the process of acquiring knowledge. Knowledge is a noun. Science is a verb.
Consider a journey in your mind into the deepest levels of your body, to the nuclear heart of your cells where the secrets of life are hidden away. Today we know that in the nucleus of every one of your cells, we can find DNA — a long ladder of matched molecules (denoted A, C, G, and T). The order of those molecules along the billion-rung DNA ladder spell out the unique information needed to create and define every single living thing on Earth. But there was a time when we didn’t know that at all. The first people to know about the the delicate double helix of this master molecule were Rosalind Franklin and her graduate student Raymond Gosling, who in 1952 took the very first picture of the molecule by bombarding it with x-rays. Today that picture is known as “Photo 51,” and its role in the discovery of DNA’s structure is storied and fraught with all too human conflicts.
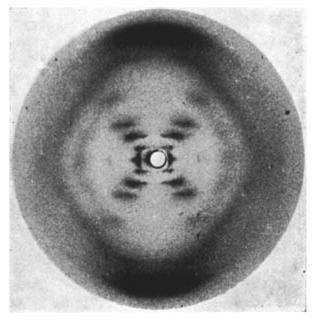
Photo 51, originally captured by Raymond Gosling and Rosalind Franklin in 1952. [Image: Wikimedia Commons]
Stare at that photo for a moment, the way scientists in the 1950’s did. Look carefully at the twist and weave, and see the rungs connecting the sides of the ladder. DNA is incomprehensibly smaller than your eye can see, but the picture captures its delicate form and spells out the previously unknown truth of how you and I and every lifeform on Earth are one with each other, siblings on the deepest levels.
Now lift your head up, and soar out to the enormously larger scale of the solar system. Voyager is NASA’s longest lived space mission, currently 45 years old and still pinging Earth with its lonely beacon as it sails beyond the Sun, farther from home than any object ever made by humans. But in early 1979 when it flew past Jupiter, it was young and in the prime of its life. It carried a suite of instruments to sense and record everything it could as it passed by the largest planet in the Sun’s family. Among the most precious things Voyager did was take pictures, and send them home to Earth like any good interstellar tourist might. Before Voyager even arrived at Jupiter, we knew about the “Great Red Spot.” It is a massive hurricane-like malestrom, twice the size of the planet Earth. It has been known to exists for the entire 400-years that humans have had telescopes and first pointed them at Jupiter; we have no idea how old it really is.
But just look at the Great Red Spot, the way Voyager did. Observe it, study it. It is exquisite in form, in shape, in complexity, in color. It almost doesn’t look real. If you saw that picture hanging on my wall, you might think it was a painting, a creative outburst of some exquisite artist here on Earth. But it is indeed a painting, a massive and beautiful canvas of chaotic color made by Nature itself.
It is easy to get swept up in the nouns of science: knowing the exact genetic code for slime molds, the chemical structures of ant pheromones, the age of the oldest crocodile fossils, the distance to the farthest quasar, the diameter of the Great Red Spot, the number of teeth a great white shark has, or the temperature at the heart of the Kilauea Volcano. These are great things science has taught us. But they are not science in and of themselves.
Science is the art of inventing ways to do the hard work of discovering. It may sound simple to figure out how many teeth a great white shark has, but it probably isn’t. It seems obvious perhaps that it is “hot” in the center of Kilauea, but I assure you no human has or could survive there. The how of getting all these bits of information, the experience of discovering, and expansion of our thinking about the world around us — that is science.
This is one of the hardest things about practicing science in the modern world: hanging on to the verbs, remembering the verbs, and giving them voice.
Science is a verb.
——————————————————-
This post is the second in a short series pondering what kinds of things make science difficult. The posts so far in this series are:
1: The Hardest Thing About Science – Language
2: The Hardest Thing About Science – Nouns & Verbs (this post)








